-
PDF
- Split View
-
Views
-
Cite
Cite
Hala Ahmadieh, Asma Arabi, Vitamins and bone health: beyond calcium and vitamin D, Nutrition Reviews, Volume 69, Issue 10, 1 October 2011, Pages 584–598, https://doi.org/10.1111/j.1753-4887.2011.00372.x
Close - Share Icon Share
Abstract
Osteoporosis is a major health disorder associated with an increased risk of fracture. Nutrition is among the modifiable factors that influence the risk of osteoporosis and fracture. Calcium and vitamin D play important roles in improving bone mineral density and reducing the risk of fracture. Other vitamins appear to play a role in bone health as well. In this review, the findings of studies that related the intake and/or the status of vitamins other than vitamin D to bone health in animals and humans are summarized. Studies of vitamin A showed inconsistent results. Excessive, as well as insufficient, levels of retinol intake may be associated with compromised bone health. Deficiencies in vitamin B, along with the consequent elevated homocysteine level, are associated with bone loss, decreased bone strength, and increased risk of fracture. Deficiencies in vitamins C, E, and K are also associated with compromised bone health; this effect may be modified by smoking, estrogen use or hormonal therapy after menopause, calcium intake, and vitamin D. These findings highlight the importance of adequate nutrition in preserving bone mass and reducing the risk of osteoporosis and fractures.
INTRODUCTION
Osteoporosis, a major health problem worldwide, is a skeletal disorder characterized by compromised bone strength, which predisposes those affected to an increased risk of fractures.
The etiology of osteoporosis is complex, as genetic constitution and modifiable factors are both known to influence the risk for low bone mass, bone loss, and fracture development.
Nutrition is one of several important modifiable factors for optimal bone health and prevention of osteoporosis. Indeed, the role of calcium and vitamin D in improving bone mineral density (BMD) and reducing fracture risk has been well established.1,–4 In addition, studies have shown that diets high in fruits and vegetables have positive effects on bone mineral status and that nutrients and vitamins, including vitamin K, vitamin C, phosphorus, potassium, magnesium, protein, and sodium, are important for the maintenance of optimal bone health.5,6 The findings of many studies have related the intake and/or serum levels of several vitamins, including A, B complex, C, E, and K, as well as the homocysteine (Hcy) level, to bone health in animals and in humans.
This review examines the available evidence regarding the role of vitamins other than vitamin D in bone health in animals and in humans. Particular attention is given to studies investigating the relationship between vitamins and BMD, and to studies assessing the possible influences of vitamins on reducing the risk of fracture.
VITAMIN A
Vitamin A is the generic term used for a group of essential fat-soluble dietary compounds, the most important ones being retinol and provitamin A (beta-carotene). Vitamin A is required for growth, reproduction, visual health, and the integrity of the immune system. Since the early 1940s, it has been recognized that vitamin A deficiency has a profound and complex effect on bone, which can lead to different types of bone abnormalities. Mellanby7 showed that vitamin A deficiency in growing animals altered both osteoclastic and osteoblastic activity, which resulted in abnormal growth in the basioccipital bone and the spine, along with serious neurological complications. A few years later, they showed that these bone changes related to vitamin A deficiencies were reversible.8 Soon after vitamin A was administered to these animals, the osteoclasts and osteoblasts became active again, resulting in the removal of the superfluous bone deposited or not absorbed during the period of vitamin A deficiency; hence, these bones tended to return to normal.8
Several studies assessed the activity of retinoids on bone cells in vitro. Dickson and Walls9 showed that collagen synthesis in embryonic chick calvaria was significantly inhibited after 24 h of culture with retinol. Others showed that in bone rudiments of mouse fetuses grown in a medium containing a high concentration of vitamin A, the terminal cartilage lost its metachromasia, shrank, and finally disintegrated.10 Studies on the effect of retinoids on osteoclast cultures yielded inconsistent results, with findings ranging from an inhibitory effect to a stimulatory effect, depending on the culture system used, the source of osteoclasts, and the species studied.11,12 Some studies showed that retinoic acid directly stimulates osteoclastic bone resorption,11 whereas others showed that retinol and retinoic acid have an inhibitory effect on osteoblastic cell proliferation in vitro.12 Conversely, studies showed that lycopene, which is a carotenoid, has a protective role because it inhibited basal and parathyroid hormone-stimulated osteoclastogenesis in rat bone marrow cultures and stimulated proliferation and differentiation of osteoblast-like osteosarcoma cells.13
Animal studies showed considerable retardation of bone growth in the fetuses of pregnant rats with hypervitaminosis A.14 Excess feeding of different species of animals with vitamin A or synthetic retinoids was associated with poor bone growth and radiolucency, accelerated bone remodeling with consequent loss of bone mineral content (BMC), and an increased rate of spontaneous fractures.15,–18 In growing rodents, this was attributed to the thinning of long bones, in which radial growth was reduced.19,20 Kneissel et al.21 showed that the long bone diameter shrank in adult rodents treated with retinoids, and they attributed this to subperiosteal osteoclastic bone resorption. Moreover, treatment of rats for 15–20 weeks with either all-trans retinoic acid or 13-cis retinoic acid reduced BMC, BMD, bone diameter, and cortical thickness of the femur, and increased the incidence of spontaneous fractures as compared to values in controls.22
The above findings from animal and in vitro studies cannot be extrapolated to humans. Nevertheless, abnormalities of ossification and calcification were described in case series in human subjects who took synthetic retinoids, especially in children who were treated for more than 4 years. The most pronounced abnormalities described in humans were osteophytic formation of the cervical spine and ossification of the atlanto-occipital ligament. In addition to modeling abnormalities, premature fusion of epiphyses and diminished bone density were observed.23,–25
Excessive intake of dietary vitamin A was negatively associated with BMD in the spine, hip, and total body in a cross-sectional study of 175 women aged 28–74 years. This effect persisted after adjustment for energy intake, level of physical activity, body mass index, smoking status, and use of estrogen.26 This negative effect of dietary vitamin A intake on BMD was not confirmed in other studies.27,–29 In the Rancho Bernardo Study, which included data from 570 women and 388 men, aged 55–92 years at baseline, there was an inverse U-shaped association of retinol intake, as assessed by food-frequency questionnaires, with baseline BMD, BMD measured 4 years later, and BMD change in the spine, total hip, and femoral neck. This association persisted after adjustment for potential osteoporosis risk factors. This association suggested that excessive as well as insufficient vitamin A intake is associated with compromised bone health.30
Several studies showed an association between vitamin A intake or retinol level and osteoporotic fractures.26,–33 In a nested case-control study of 247 women aged 40–76 years and 873 age-matched controls, a high intake of dietary retinol was associated with an increased risk of hip fractures. In this study, for every 1-mg increase in the daily intake of retinol, the risk of hip fracture was further increased by 68%.26 This result was confirmed by the Nurses' Health Study, which included a total of 72,337 postmenopausal women aged 34–77 years in whom long-term intake of a diet high in retinol was associated with an increased risk of hip fracture. This association was attenuated among women on estrogen therapy.31 In this study, there was a nonsignificant increase in the risk of hip fracture among women using vitamin A supplements compared with women who did not use supplements. Conversely, in the Iowa Women's Health Study, which included 34,703 postmenopausal women who were followed up for 9.5 years, supplemental, but not dietary, vitamin A intake was associated with an increased risk of fracture, but this relationship was not dose dependent.32
The relationship between the risk of fracture and vitamin A status, assessed as serum retinol levels, was also evaluated. In a prospective study of 2,322 men aged 49–51 years and followed up for 30 years, the relative risk of hip fracture was 2.4 in the group with the highest serum retinol level (75.62 µg/dL) as compared with that in subjects whose retinol levels were between 62.16 µg/dL and 67.60 µg/dL.33 Conversely, case-control studies suggested a negative relationship between serum retinol level and vertebral fractures in women.34 Interestingly, the association between vitamin A and fracture was attributed mostly to retinol, but beta-carotene intake and/or levels were not associated with fracture risk.31,33
On the other hand, some studies evaluated the association between retinol levels and markers of bone turnover. A cross-sectional study of postmenopausal women aged 50–60 years showed that higher lycopene intake, as assessed by dietary records, was associated with lower levels of cross-linked N-telopeptides of type I collagen and other bone turnover markers and hence may have a beneficial effect in reducing the risk of osteoporosis.35 Unlike vitamin A intake from dietary sources, vitamin A supplementation was not associated with changes in serum markers of bone turnover in a prospective, randomized, single-blinded study of 80 healthy men aged 18–58 years.29
In conclusion, studies evaluating the association between serum retinol level or retinol intake and skeletal health in humans showed inconsistent results. This inconsistency may be related to the difficulty in obtaining an accurate assessment of the different methods used to estimate vitamin A intake, and to the use of serum retinol level, which is an unstable marker and a poor indicator of vitamin A status. Rebaya-Mercado and Blumberg36 recommended the use of retinyl esters rather than retinol in future studies assessing vitamin A status. Further studies are needed to determine the safest dose of vitamin A that does not have a deleterious effect on skeletal health. Until additional evidence is available, it is important to ensure sufficient vitamin A supplementation, especially in children, in whom vitamin A deficiency occurs worldwide (Table 1).
Relevant studies examining the relationships between vitamin A intake or serum retinol levels and bone health.
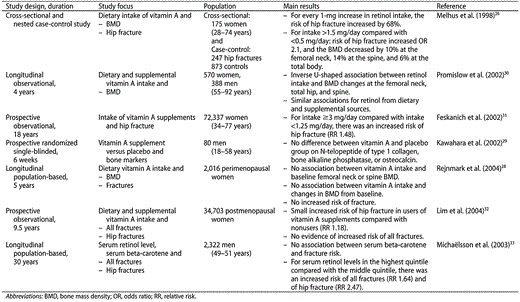
Relevant studies examining the relationships between vitamin A intake or serum retinol levels and bone health.

VITAMIN B COMPLEX
One of the potentially modifiable risk factors for osteoporotic fracture is an elevated Hcy level.37,38 Increased plasma Hcy concentrations result from defective clearance or from defects in intracellular Hcy metabolism. These metabolic defects can have a genetic or nutritional origin.39,–41 Vitamin B, including B2 (riboflavin), B6 (pyridoxine), B11 (folate), and B12 (cobalamin), serve as cofactors or substrates for the enzymes involved in Hcy metabolism and are therefore determinants of Hcy status.40,–43
A link between Hcy level and skeletal abnormality was first noticed in studies of homocysteinuria, a classic metabolic disorder caused by the deficiency of cystathionine beta-synthase, the first enzyme involved in Hcy trans-sulfuration.43 In vitro studies attributed the cause of osteoporosis in these patients to impaired cross-linking of collagen.44 In vitro studies performed later showed that Hcy-thiolactone, a natural metabolite of Hcy, inhibits lysyl oxidase, an enzyme involved in collagen cross-linking, and that increased concentrations of Hcy itself stimulate osteoclast activity in vitro.45,–47 Moreover, a number of studies showed that cobalamin (vitamin B12), an important determinant of Hcy levels, had a stimulatory effect on the expression of alkaline phosphatase in human-rib-derived osteoprogenitors, in rat osteosarcoma cells, and in chicken calvaria-derived osteoblasts.48,49 Animal studies showed a 40% reduction in bone strength and a drastic 90% removal of spongious bone matrix after 3 months of induced hyperhomocysteinemia in adult rats.50 Other studies in mice showed that pyridoxine (vitamin B6) deficiency, which also leads to increased Hcy levels, resulted in impaired cross-link formation in bone.51
Several studies in humans revealed an association between Hcy levels and markers of bone resorption and BMD. In a study of 328 postmenopausal British women, osteoporotic patients had a significantly higher Hcy level compared with nonosteoporotic patients.52 High Hcy levels were also associated with an increased rate of bone loss in the hip in a cohort of 1,213 women aged 70–85 years who were followed up for 4 years. This effect persisted after accounting for bone size, dietary nutrient intakes, physical activity, and renal function.53 The correlation between Hcy and BMD in postmenopausal women, however, was not confirmed by others.54,–56
In addition to investigating the relationship between Hcy levels and BMD, several studies assessed the relationship between BMD and the determinants of Hcy levels, such as riboflavin, pyridoxine, cobalamin, folate, and methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms.
An association between MTHFR gene polymorphisms and BMD was reported in postmenopausal Japanese women. Women who were homozygous for the thermolabile TT variant had a lower BMD in the lumbar spine and total body compared with women with the CC and CT genotypes.57 Similar findings were reported in the Danish Osteoporosis Prevention Study and the Framingham Osteoporosis Study.58,59 In the latter study, this association between MTHFR gene polymorphisms and BMD was dependent on folate status.60
Several epidemiological studies have shown a positive association between folate and/or cobalamin status and bone endpoints.56,–64 In the British study, folate was an independent predictor of BMD after adjustment for age, weight, and height.52 Conversely, the third US National Health and Nutrition Examination Survey did not show an association between serum or red blood cell folate levels and BMD in 1,550 elderly Americans.62 This controversy could be related to the older age of the population studied and the higher mean plasma folate concentration in that population. In the US National Health and Nutrition Examination Survey, serum vitamin B12 was related to BMD.62 This correlation between B12 concentrations and BMD was also reported by others.59,–63 In the Framingham Osteoporosis Study, a low vitamin B12 concentration was associated with lower hip BMD in men and lower spine BMD in women59; conversely, marginal or deficient vitamin B12 levels were associated with an increased risk of osteoporosis in elderly Dutch women but not in men.63 A longitudinal study showed that low serum vitamin B12 concentration was associated with an increased annual rate of bone loss in the hip but not in the calcaneum in postmenopausal elderly women.64 However, the relationship between B12 and bone density was not consistent in all studies.52,56
Just as with vitamin B12, a significant correlation between vitamins B2 and B6 and BMD has been reported.65 In a large cohort of subjects aged 55 years and older from the Rotterdam Study, a small but significant correlation between dietary intake of riboflavin and pyridoxine and femoral neck BMD was found, with a decreased risk of nonvertebral fractures. This relationship persisted after adjustment for comorbidities and dietary intake of other vitamins, protein, calcium, and vitamin D.65 In a longitudinal study of 1,241 Scottish women aged 45–54 years and followed up for a mean of 6.6 years, there was no significant association between BMD and either MTHFR genotype or B complex vitamins when examined separately. However, there was a significant interaction between riboflavin intake, MTHFR TT genotype, and BMD, suggesting that riboflavin intake and the MTHFR genotype might interact to regulate BMD.66
In addition to their relationship with BMD, Hcy levels and B complex deficiencies have also been associated with fracture risk. Van Meurs et al.37 found a strong relationship between Hcy level and fracture incidence in 2,406 subjects aged 55 years and older who participated in two prospective, population-based studies, the Rotterdam Study and the Longitudinal Aging Study. In these studies, there were three independent cohorts of subjects followed up for a mean duration of 2.7–8.1 years. Although there was no relationship between Hcy level and BMD, an Hcy level in the highest quartile was associated with a double risk of fracture, independent of BMD and other potential risk factors for fracture. The risk was similar in all three cohorts studied, and it was similar in men and women.37 Similarly, in 1,267 subjects in the Longitudinal Aging Study Amsterdam who were followed up for 3 years, Dhonukshe-Rutten et al.67 showed that low B12 concentrations and high Hcy levels were associated with an increased risk of fracture, with a relative risk of 3.8 in men and 2.8 in women. This relationship persisted after adjustment for age, sex, body weight, body height, current smoking habits, mobility, and cognition. These relative risks were similar to those shown in the Framingham Study, which enrolled 825 men and 1,174 women aged 59–91 years. Men and women in the highest quartile of Hcy levels had a greater risk of hip fracture than those in the lowest quartile. The risk was almost four times as high for men and twice as high for women.38 Important evidence was also obtained from a Japanese intervention study, which showed a 70% reduction in the risk of fracture in stroke patients after Hcy levels were lowered. That study, however, was not extended to other patients with a high fracture risk.68
On the other hand, the Danish Osteoporosis Prevention Study showed that the MTHFR gene polymorphism was also associated with an increased risk of fracture. TT individuals had a twofold increased risk of fracture when compared with those with other genotypes.58 This finding, however, was not confirmed in a study of Chinese men and women.69 Pyridoxine intake was associated with significantly decreased fracture risk, and this relationship was independent of Hcy levels.65 Moreover, a correlation was found between decreased circulating pyridoxine concentrations and impaired cross-link formation in bone of human individuals with fracture.65
Overall, studies showed evidence of a relationship between beta-complex intake and levels and bone health. Although defects in beta-complex metabolism may have a genetic origin, it is possible that low vitamin B levels may be of nutritional origin. Therefore, an interaction between these low levels/intake and/or the intake/levels of other nutrients that influence bone health cannot be excluded (Table 2).
Relevant studies examining the relationships between vitamin B complex and bone health.
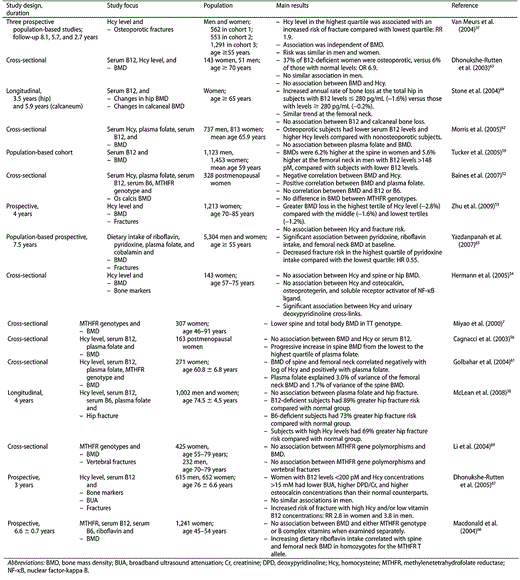
Relevant studies examining the relationships between vitamin B complex and bone health.

VITAMIN C
Ascorbic acid (vitamin C), an essential vitamin that humans are unable to synthesize, is required for the hydroxylation of lysine and proline, which are needed for the formation of stable collagen triple helixes and, therefore, normal bone development.70
Studies of different animal and human tissues suggested that ascorbic acid stimulates alkaline phosphatase activity and is required for the formation of type I collagen matrix as well as for the expression of osteoblastic markers and mineralization.71,–73 Scurvy, a disease caused by severe ascorbic acid deficiency, was associated with decreased BMD and BMC in guinea pigs, especially during the phases of skeletal maturation.74 This negative effect on peak bone mass persisted in these pigs in adult life. Associated bone abnormalities included thinner growth plate, thinner trabeculae, increased bone resorption, decreased differentiation of osteoblasts from mesenchymal cells, and decreased collagen synthesis in the proximal tibial epiphysis.74 Moreover, a low- or non-ascorbic acid diet resulted in reduced femur calcium and hydroxyproline contents, low collagen formation, low femoral bone density, and abnormal cartilage growth morphology of the proximal tibial metaphysis of these animals.75
In humans, the Postmenopausal Estrogen/Progestin Interventions Trial found a positive association between vitamin C intake and BMD of the spine and hip. This association was modified by the level of dietary calcium intake but was independent of the intake of other nutrients.76 This association between dietary vitamin C intake and BMD was confirmed by some,77,–79 but not all, studies.80,81 In the Women's Health Initiative Study, although there was no significant association between vitamin C intake and BMD, the beneficial effect of hormone treatment on BMD at all skeletal sites was stronger with higher intakes of vitamin C.80 In the Framingham Osteoporosis Study, vitamin C intake was associated with femoral neck BMD among male nonsmokers,82 but in contrast to the Postmenopausal Estrogen/Progestin Interventions Trial, the significant association was found among men with low calcium intake but not among those with high calcium intake. After 4 years of follow up, both supplemental and dietary vitamin C intake were associated with less BMD loss in the hip, spine, and radial shaft. This association was most evident in men with low calcium and low vitamin E intakes and was greater in those who obtained vitamin C from diet as opposed to supplements.82
Thus, there is a positive but complex association between vitamin C intake and bone density, which may be related to the interaction of other factors like smoking, estrogen use or hormonal therapy after menopause, calcium intake, and vitamin E intake (Table 3).
Relevant studies examining the relationships between antioxidants and bone health.
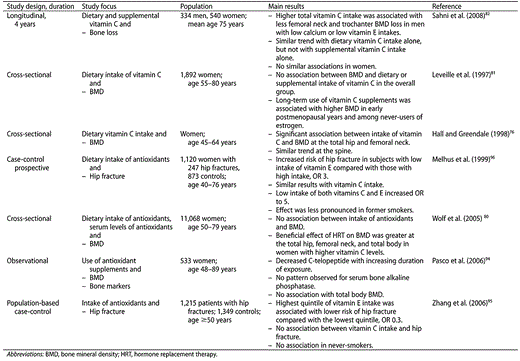
Relevant studies examining the relationships between antioxidants and bone health.

VITAMIN E
Vitamin E is an important fat-soluble vitamin with essential antioxidant properties.83 There are two types of vitamin E, tocopherol and tocotrienol. Recently, tocotrienol has gained increasing scientific interest because of its high antioxidative activity. Oxidative stress is a state of excess free radical formation. Free radicals are involved in the apoptosis of osteoblasts and osteocytes and in osteoclastogenesis and, therefore, in bone resorption, as shown in different in vitro and animal studies.84 In addition, oxidative stress increases bone resorption through activation of nuclear factor-kappa B, which normally regulates osteoclast differentiation and, thus, bone resorption and remodeling.85
An inhibitory effect of vitamin E on collagen production has been reported in different rodent tissues.86 Other studies, however, found that vitamin E increased hepatic hydroxyproline content in rabbits and partially restored collagen synthesis in primary cultures of avian epiphyseal chondrocytes.87 In suckling lambs, intramuscular injections of vitamin E had a protective effect on membranes of chondrocytes during maturation and differentiation.88 It has been suggested that vitamin E inhibits cartilage resorption and protects chondrocyte membranes through reduction of free radical generation and lipid peroxidation.89 Schwartz90 reported that vitamin E has a positive effect on the stabilization of sulfated proteoglycans, which are incorporated into the collagen matrix. Maniam et al.91 confirmed that vitamin E, especially tocotrienols, had antiosteoporotic activities and improved the trabecular structural and cellular properties in different animal models subjected to ovariectomy, orchidectomy, thyroidectomy, and oxidative stress.
A link between increased oxidative stress and reduced BMD has been documented in humans.92 Alpha-tocopherol prevented osteoclast formation and bone loss in elderly men by inhibiting both RANK ligand inductions in osteoblasts and c-Fos expression in osteoclast precursors.93 An observational study of 533 randomly selected nonsmoking postmenopausal women showed that the duration of use of antioxidant supplements, including vitamins C and E, negatively correlated with age-adjusted and weight-adjusted serum C-telopeptide, the marker of bone resorption, thus suggesting that antioxidant vitamins may suppress bone resorption in humans.94
Few studies have examined the relationship between antioxidant intake and risk of hip fracture.94,–96 A nested case-control study in the Swedish Mammography Cohort revealed that low intake of vitamins C and E increased the risk of hip fracture in smokers.94 Similarly, a large case-control study that included data on hip fractures in more than 1,000 male and female patients aged 50 years or older found an inverse dose-response association between intake of vitamin E and risk of hip fracture. This association was found in current and ex-smokers but not in never-smokers.95 Similarly, Melhus et al.96 prospectively studied 66,651 women aged 40–76 years. After 64 months, the odds ratio for hip fracture in smokers was 3 for low intake of vitamin E. The odds ratio increased to 4.9 in smokers with low intakes of both vitamins E and C. These relationships were less pronounced in former smokers. The mechanism by which higher intakes of antioxidants were associated with a lower risk of hip fracture in smokers is unclear; however, smoking by itself is a risk factor for bone loss and fracture,97,–99 and this diverse effect of smoking on bone health has been postulated to be due, in part, to the oxidative stress caused by smoking.86
In conclusion, vitamin E seems to be associated with increased bone mass and decreased fracture risk in humans. This relationship is modulated by smoking status and is mediated primarily by the antioxidant properties of vitamin E.
VITAMIN K
Vitamin K was originally identified as an essential factor for blood coagulation. However, it was also found to have multiple other functions, and there is emerging evidence that vitamin K may have a protective role against age-related bone loss.100 This is mediated mainly through the vitamin-K-dependent gamma-carboxylation of osteocalcin. Over 40 years ago, Price et al.101 and Price and Nishimoto102 demonstrated that bone Gla protein (osteocalcin) is an excellent marker of bone metabolism. They discovered that bone Gla protein is present in serum and plasma, and they developed a radioimmunoassay to measure it. They also showed that this bone-specific protein synthesized by osteoblasts undergoes vitamin-K-dependent gamma-carboxylation. Undercarboxylated osteocalcin increases with vitamin K insufficiency and with administration of anti-vitamin K because it affects the gamma-carboxylation.103 Others attributed the antiresorptive effects of vitamin K to its geranylgeranyl side chain via mechanisms independent of gamma-carboxylation. Other suggested mechanisms of action of vitamin K on bone included an enhancement of collagen accumulation by activation of the steroid and xenobiotic receptor.104 Moreover, vitamin K may influence bone metabolism through its effect on urinary calcium excretion105 or by inhibiting the production of certain bone-resorbing agents such as prostaglandin E2 and interleukin 6.106
Animal data suggested that vitamins K and D work synergistically on bone metabolism.107,108 Indeed, a study in ovariectomized rats showed that ovariectomy-induced bone loss was reduced in the group that received both vitamins K and D, but not in the groups that received vitamin K alone or vitamin D alone.108 On the other hand, it has been shown that treatment with vitamin K improved bone strength more than BMD in these animals.107,108 Indeed, magnesium-insufficient bone is fragile upon mechanical loading, but vitamin K improved both the maximum load and the elastic modulus without influencing BMC in rats fed a low-magnesium diet.109
In humans, vitamin K intake and vitamin K status were not related to the biochemical markers of bone metabolism or to BMD in healthy young subjects.110 In postmenopausal women, vitamin K supplementation increased total and carboxylated osteocalcin, decreased uncarboxylated osteocalcin, and decreased urinary calcium and hydroxyproline.111 BMD data in postmenopausal women were controversial. Vermeer et al.112 showed that vitamin K intake was positively associated with BMD at many different sites. Braam et al.113 randomized 181 postmenopausal women aged 50–60 years to receive a daily placebo, minerals (calcium, magnesium, zinc, and vitamin D) only, or minerals and vitamin D and phylloquinone (vitamin K1). After 3 years, the group receiving the combination of minerals, vitamin D, and K1 had lower bone loss at the femoral neck but not at the lumbar spine. However, other randomized, double-blind, placebo-controlled trials did not support a role for vitamin K supplementation in osteoporosis prevention in postmenopausal women.114,–116 In a large double-blind placebo-controlled study, 381 North American postmenopausal women were randomized to phylloquinone (vitamin K1), menatetrenone, or placebo for 1 year. At the end of the study, treatment reduced undercarboxylated osteocalcin compared to placebo, but there was no significant effect of K1 or menatetrenone on serum bone alkaline phosphatase, N-telopeptide of type 1 collagen, spine or hip BMD, calcaneal ultrasound parameters, or femur geometry properties as measured by dual-emission X-ray absorptometry.116
The first report linking vitamin K serum levels to osteoporotic fractures dates back to 1985. Hart et al.117 demonstrated that patients who had sustained an acute hip fracture or suffered from vertebral compression fracture had lower circulating vitamin K serum levels compared with control subjects. These findings were confirmed by others.118,–121 The effect of vitamin K intake on fracture risk was assessed in the Nurses' Health Study. In this prospective analysis of data from 72,327 women aged 34–77 years followed up over 10 years, low dietary intake of vitamin K was associated with an increased risk of hip fracture after adjustment for calcium and vitamin D intake.122 In the Framingham Heart Study, which included 335 men and 553 women aged 75 years, there was no association between dietary phylloquinone intake and BMD in either men or women, but women within the highest quartile of vitamin K intake had a significantly lower risk of hip fracture than those in the lowest quartile of intake.123
Finally, the effect of supplemental vitamin K on reducing fracture risk was addressed in several randomized trials. In a randomized open-label trial that included 241 osteoporotic patients, treatment with vitamin K2 was associated with reduced fracture occurrence, although there was no change in lumbar spine BMD or in urinary deoxypyridinoline excretion in the treated group.124 Similarly, small, randomized controlled trials and post-hoc analyses of a large randomized controlled trial in Japanese patients indicated that a large daily dose of vitamin K (45 mg/day given as menaquinone-4) for 12–24 months had a beneficial effect on reducing hip, vertebral, and nonvertebral fractures.125
In conclusion, although studies have shown that low circulating levels and/or low dietary intake was associated with low bone density and with increased fracture risk in humans, a protective effect of vitamin K supplementation was not confirmed in randomized controlled trials. These discrepancies may be due, in part, to the collinearity between intake of vitamin K and intake of other nutrients. Although vitamin K level is an excellent biochemical marker of vitamin K status, it does not necessarily reflect a subject's overall nutritional status. Dietary and nutritional patterns may be more important than the intake or level of individual nutrients (Table 4).
Relevant studies examining relationships between vitamin K and bone health parameters.
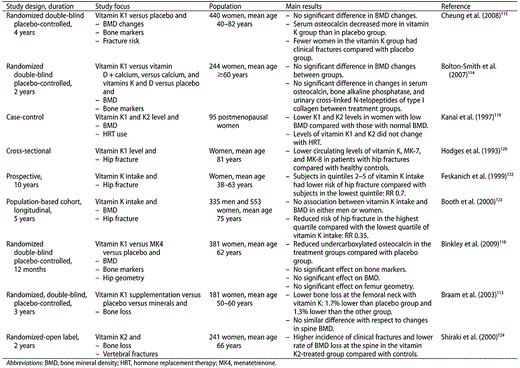
Relevant studies examining relationships between vitamin K and bone health parameters.

CONCLUSION
The available data provide clear evidence that the effects of nutrition on bone health are not limited to those resulting from calcium and vitamin D intake. This review highlights the importance of nutritional factors in preventing and reducing the risk of osteoporosis and fractures.
Both excessive and insufficient intake of retinol may be associated with compromised bone health; however, data on vitamin A intake from dietary sources and from supplemental intake, as well as data on vitamin A status determined by serum retinol levels, showed inconsistent results. Most, but not all, studies showed that vitamin B complex and vitamins C, E, and K correlated positively with BMD at multiple skeletal sites and/or were associated with reduced risk of fracture, independent of BMD. Therefore, nutrients may have adverse effects on bone strength in a variety of different ways, not just by affecting bone mass.
The relationship between vitamins other than vitamin D on bone is complex and seems to be affected by genetic factors, gender, menopausal status, hormonal therapy, smoking, and calcium intake. It is possible that nutrient patterns, and not individual foods or vitamins, are important in bone health, thus explaining some of the paradoxical results related to individual nutrients. Indeed, all tissues need all nutrients, and bone should not be an exception. Therefore, well-balanced and adequate nutrition should be ensured in order to prevent adverse effects on bone health.
Acknowledgments
Declaration of interest
The authors have no relevant interests to declare.
REFERENCES



